2026

Brett W. Stringer, Yougang Zhang, Afsaneh Taghipour-Sheshdeh, Shuxiang Goh, Heike Kölbel, Michelle A Farrar, Brunhilde Wirth, Jean Giacomotto
Clinical relevance of zebrafish for gene variants testing. Proof-of-principle with SMN1/SMA Journal Article
In: EMBO Molecular Medicine – FRONT COVER, vol. 18, iss. 1, 2026.
@article{nokey,
title = {Clinical relevance of zebrafish for gene variants testing. Proof-of-principle with SMN1/SMA},
author = {Brett W. Stringer, Yougang Zhang, Afsaneh Taghipour-Sheshdeh, Shuxiang Goh, Heike Kölbel, Michelle A Farrar, Brunhilde Wirth, Jean Giacomotto},
url = {https://link.springer.com/article/10.1038/s44321-025-00355-8},
doi = {10.1038/s44321-025-00355-8},
year = {2026},
date = {2026-01-15},
journal = {EMBO Molecular Medicine - FRONT COVER},
volume = {18},
issue = {1},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2025
Fangfei Guo, Alisha Tromp, Haitao Wang, Thomas E Hall, Jean Giacomotto
Cre-Lox miRNA-delivery technology optimized for inducible microRNA and gene-silencing studies in zebrafish Journal Article
In: Nucleic Acids Research (NAR), 2025.
@article{nokey,
title = {Cre-Lox miRNA-delivery technology optimized for inducible microRNA and gene-silencing studies in zebrafish},
author = {Fangfei Guo, Alisha Tromp, Haitao Wang, Thomas E Hall, Jean Giacomotto },
doi = {10.1093/nar/gkaf004},
year = {2025},
date = {2025-01-11},
urldate = {2025-01-11},
journal = {Nucleic Acids Research (NAR)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Brett W. Stringer, Yougang Zhang, Afsaneh Taghipour-Sheshdeh, Shuxiang Goh, Heike Kölbel, Michelle A Farrar, Brunhilde Wirth, Jean Giacomotto
Clinical relevance of zebrafish for gene variants testing. Proof-of-principle with SMN1/SMA Journal Article
In: biorxiv, 2025.
@article{nokey,
title = {Clinical relevance of zebrafish for gene variants testing. Proof-of-principle with SMN1/SMA},
author = {Brett W. Stringer, Yougang Zhang, Afsaneh Taghipour-Sheshdeh, Shuxiang Goh, Heike Kölbel, Michelle A Farrar, Brunhilde Wirth, Jean Giacomotto},
url = {https://www.biorxiv.org/content/10.1101/2025.01.30.632288v1},
year = {2025},
date = {2025-01-31},
urldate = {2025-01-31},
journal = {biorxiv},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Gengxuan Shi, Yaoying Lu, Yougang Zhang, Ke Zheng, Jean Giacomotto, Kathryn F. Tonissen, Yunjiang Feng
Isolation and Biological Evaluation of Human Tyrosinase Inhibitors from the Fruit of Xanthium strumarium L Journal Article
In: Molecules, 2025.
@article{nokey,
title = {Isolation and Biological Evaluation of Human Tyrosinase Inhibitors from the Fruit of Xanthium strumarium L},
author = {Gengxuan Shi, Yaoying Lu, Yougang Zhang, Ke Zheng, Jean Giacomotto, Kathryn F. Tonissen and Yunjiang Feng},
doi = {10.3390/molecules30183689},
year = {2025},
date = {2025-08-08},
urldate = {2025-08-08},
journal = {Molecules},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
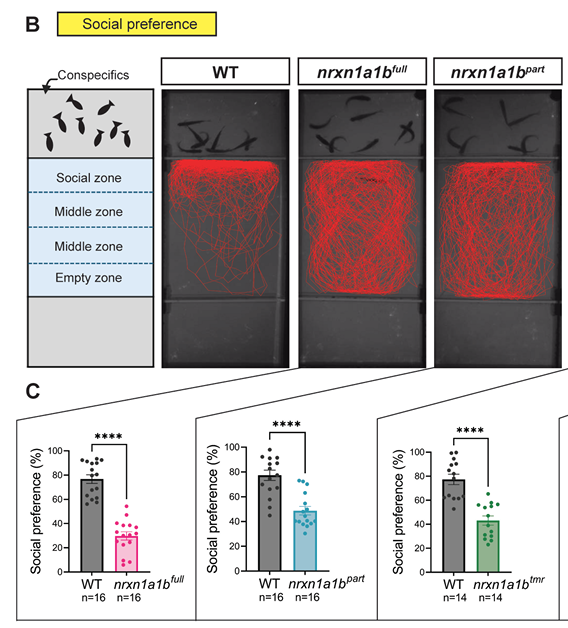
Q Nguyen, F Guo, B Mowry, J Das, J Giacomotto
Modelling mental disorders in zebrafish. Neurexins severely modulate anxiety, social behaviours and aggression Journal Article
In: bioRxiv, 2025.
@article{nokey,
title = {Modelling mental disorders in zebrafish. Neurexins severely modulate anxiety, social behaviours and aggression},
author = {Q Nguyen, F Guo, B Mowry, J Das, J Giacomotto},
url = {https://www.biorxiv.org/content/10.1101/2025.11.24.690344v1},
year = {2025},
date = {2025-11-25},
journal = {bioRxiv},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2024
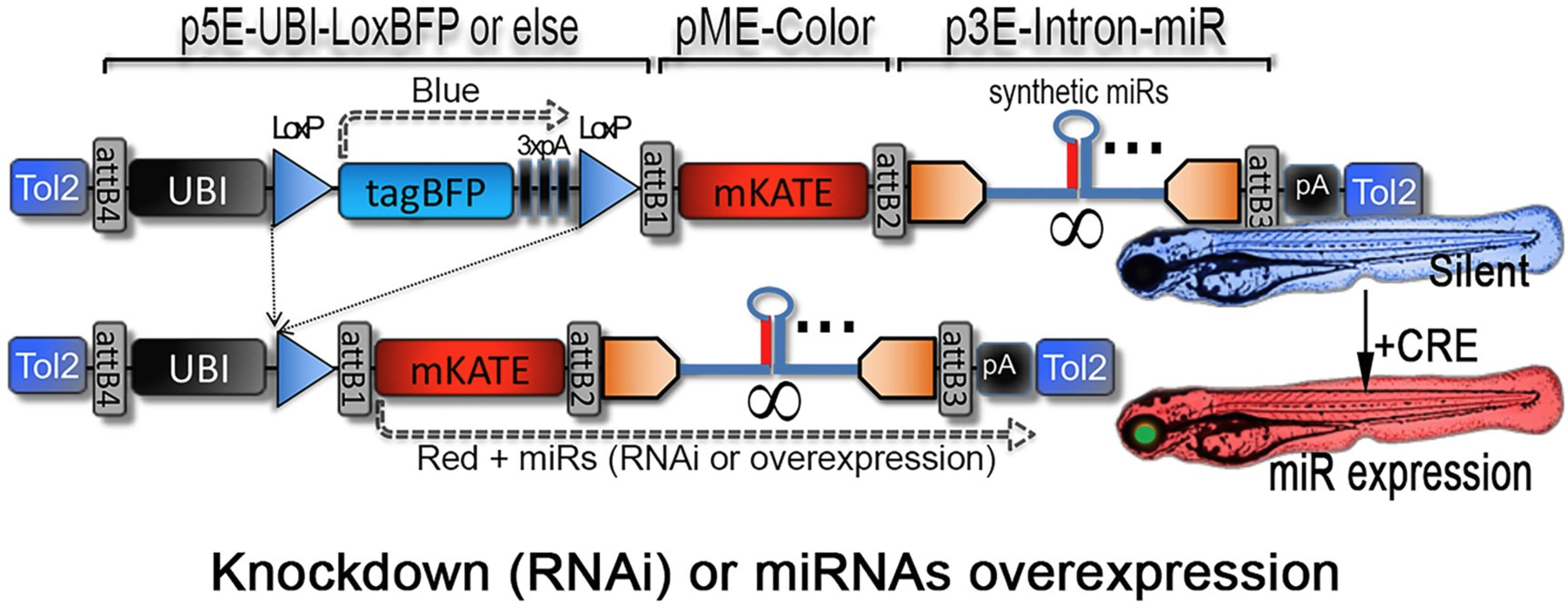
Guo F., Tromp A., Wang H., Hall T., Giacomotto J.
Cre-Lox miRNA-delivery technology optimized for inducible microRNA and gene-silencing studies in zebrafish Journal Article
In: BioRxiv, 2024.
@article{nokey,
title = {Cre-Lox miRNA-delivery technology optimized for inducible microRNA and gene-silencing studies in zebrafish},
author = {Guo F., Tromp A., Wang H., Hall T., Giacomotto J.},
doi = {https://www.biorxiv.org/content/10.1101/2024.02.22.581357v1},
year = {2024},
date = {2024-02-22},
urldate = {2024-02-22},
journal = {BioRxiv},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Kaiyrzhanov R, Ortigoza-Escobar JD, Stringer BW, Ganieva M, Gowda VK, Srinivasan VM, Macaya A, Laner A, Onbool E, Al-Shammari R, Al-Owain M, Deconinck N, Vilain C, Dontaine P, Self E, Akram R, Hussain G, Baig SM, Iqbal J, Salpietro V, Neshatdoust M, Kasiri M, Yesil G, Uygur T, Pysden K, Berry IR, Alves CA, Giacomotto J, Houlden H, Maroofian R.
Clinical and Molecular Spectrum of Autosomal Recessive CA8-Related Cerebellar Ataxia Journal Article
In: Movement Disorder, 2024.
@article{nokey,
title = {Clinical and Molecular Spectrum of Autosomal Recessive CA8-Related Cerebellar Ataxia},
author = {Kaiyrzhanov R, Ortigoza-Escobar JD, Stringer BW, Ganieva M, Gowda VK, Srinivasan VM, Macaya A, Laner A, Onbool E, Al-Shammari R, Al-Owain M, Deconinck N, Vilain C, Dontaine P, Self E, Akram R, Hussain G, Baig SM, Iqbal J, Salpietro V, Neshatdoust M, Kasiri M, Yesil G, Uygur T, Pysden K, Berry IR, Alves CA, Giacomotto J, Houlden H, Maroofian R.},
doi = {10.1002/mds.29754},
year = {2024},
date = {2024-04-09},
urldate = {2024-04-09},
journal = {Movement Disorder},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2023
Tromp A., Wang H., Hall TE, Mowry B., Giacomotto J.
Optimising the zebrafish Cre/Lox toolbox. Codon improved iCre, new gateway tools, Cre protein and guidelines. Journal Article
In: Frontiers in Physiology, 2023.
@article{nokey,
title = {Optimising the zebrafish Cre/Lox toolbox. Codon improved iCre, new gateway tools, Cre protein and guidelines. },
author = {Tromp A., Wang H., Hall TE, Mowry B., Giacomotto J.},
doi = {10.3389/fphys.2023.1221310},
year = {2023},
date = {2023-08-03},
urldate = {2023-08-03},
journal = {Frontiers in Physiology},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Hall TE, Ariotti N, Lo HP, Rae J, Ferguson C, Martel N, Lim YW, Giacomotto J, Parton RG.
Flexing fish: cell surface plasticity in response to shape change in the whole organism. Journal Article
In: Current Biology, 2023.
@article{nokey,
title = {Flexing fish: cell surface plasticity in response to shape change in the whole organism. },
author = {Hall TE, Ariotti N, Lo HP, Rae J, Ferguson C, Martel N, Lim YW, Giacomotto J, Parton RG.},
doi = {10.1016/j.cub.2023.08.068},
year = {2023},
date = {2023-10-11},
urldate = {2023-10-11},
journal = {Current Biology},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2022
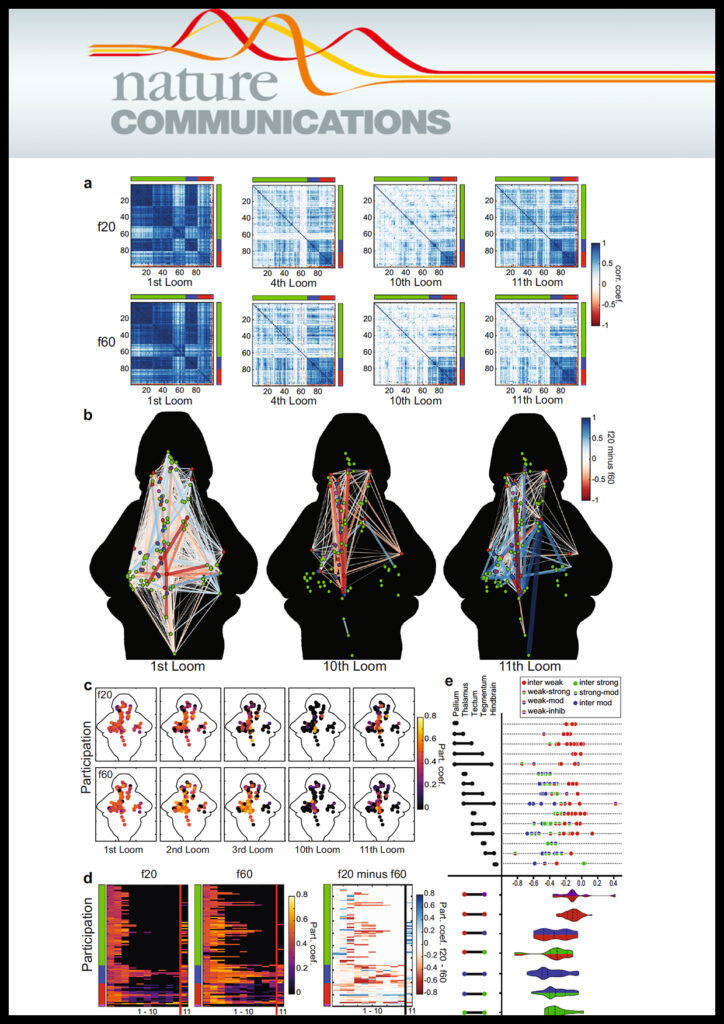
Emmanuel Marquez-Legorreta, Lena Constantin, Marielle Piber, Itia A Favre-Bulle, Michael A Taylor, Ann S Blevins, Jean Giacomotto, Dani S Bassett, Gilles C Vanwalleghem, Ethan K Scott
Brain-wide visual habituation networks in wild type and fmr1 zebrafish Journal Article
In: Nat Commun, vol. 13, no. 1, pp. 895, 2022, ISSN: 2041-1723.
@article{pmid35173170,
title = {Brain-wide visual habituation networks in wild type and fmr1 zebrafish},
author = {Emmanuel Marquez-Legorreta and Lena Constantin and Marielle Piber and Itia A Favre-Bulle and Michael A Taylor and Ann S Blevins and Jean Giacomotto and Dani S Bassett and Gilles C Vanwalleghem and Ethan K Scott},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2022-fmr1-zebrafish-Nat-Comm.pdf},
doi = {10.1038/s41467-022-28299-4},
issn = {2041-1723},
year = {2022},
date = {2022-01-01},
urldate = {2022-01-01},
journal = {Nat Commun},
volume = {13},
number = {1},
pages = {895},
abstract = {Habituation is a form of learning during which animals stop responding to repetitive stimuli, and deficits in habituation are characteristic of several psychiatric disorders. Due to technical challenges, the brain-wide networks mediating habituation are poorly understood. Here we report brain-wide calcium imaging during larval zebrafish habituation to repeated visual looming stimuli. We show that different functional categories of loom-sensitive neurons are located in characteristic locations throughout the brain, and that both the functional properties of their networks and the resulting behavior can be modulated by stimulus saliency and timing. Using graph theory, we identify a visual circuit that habituates minimally, a moderately habituating midbrain population proposed to mediate the sensorimotor transformation, and downstream circuit elements responsible for higher order representations and the delivery of behavior. Zebrafish larvae carrying a mutation in the fmr1 gene have a systematic shift toward sustained premotor activity in this network, and show slower behavioral habituation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
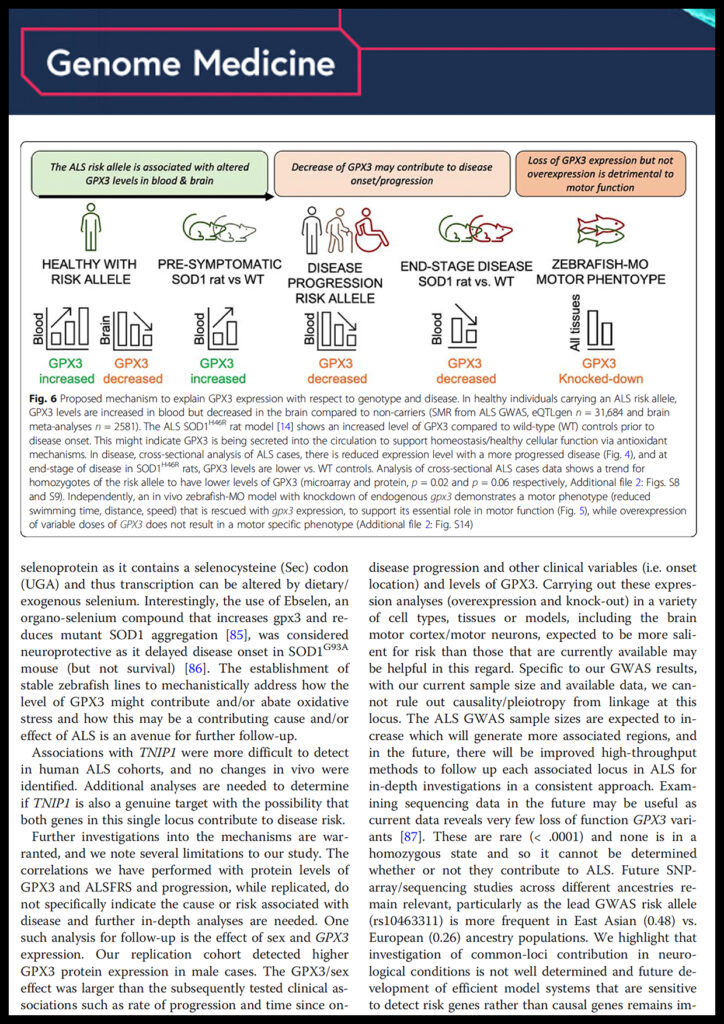
Restuadi Restuadi, Frederik J Steyn, Edor Kabashi, Shyuan T Ngo, Fei-Fei Cheng, Marta F Nabais, Mike J Thompson, Ting Qi, Yang Wu, Anjali K Henders, Leanne Wallace, Chris R Bye, Bradley J Turner, Laura Ziser, Susan Mathers, Pamela A McCombe, Merrilee Needham, David Schultz, Matthew C Kiernan, Wouter van Rheenen, Leonard H van den Berg, Jan H Veldink, Roel Ophoff, Alexander Gusev, Noah Zaitlen, Allan F McRae, Robert D Henderson, Naomi R Wray, Jean Giacomotto, Fleur C Garton
Functional characterisation of the amyotrophic lateral sclerosis risk locus GPX3/TNIP1 Journal Article
In: Genome Med, vol. 14, no. 1, pp. 7, 2022, ISSN: 1756-994X.
@article{pmid35042540,
title = {Functional characterisation of the amyotrophic lateral sclerosis risk locus GPX3/TNIP1},
author = {Restuadi Restuadi and Frederik J Steyn and Edor Kabashi and Shyuan T Ngo and Fei-Fei Cheng and Marta F Nabais and Mike J Thompson and Ting Qi and Yang Wu and Anjali K Henders and Leanne Wallace and Chris R Bye and Bradley J Turner and Laura Ziser and Susan Mathers and Pamela A McCombe and Merrilee Needham and David Schultz and Matthew C Kiernan and Wouter van Rheenen and Leonard H van den Berg and Jan H Veldink and Roel Ophoff and Alexander Gusev and Noah Zaitlen and Allan F McRae and Robert D Henderson and Naomi R Wray and Jean Giacomotto and Fleur C Garton},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2022-GPX3-Genome-Medicine-Final-2.pdf},
doi = {10.1186/s13073-021-01006-6},
issn = {1756-994X},
year = {2022},
date = {2022-01-01},
urldate = {2022-01-01},
journal = {Genome Med},
volume = {14},
number = {1},
pages = {7},
abstract = {BACKGROUND: Amyotrophic lateral sclerosis (ALS) is a complex, late-onset, neurodegenerative disease with a genetic contribution to disease liability. Genome-wide association studies (GWAS) have identified ten risk loci to date, including the TNIP1/GPX3 locus on chromosome five. Given association analysis data alone cannot determine the most plausible risk gene for this locus, we undertook a comprehensive suite of in silico, in vivo and in vitro studies to address this.
METHODS: The Functional Mapping and Annotation (FUMA) pipeline and five tools (conditional and joint analysis (GCTA-COJO), Stratified Linkage Disequilibrium Score Regression (S-LDSC), Polygenic Priority Scoring (PoPS), Summary-based Mendelian Randomisation (SMR-HEIDI) and transcriptome-wide association study (TWAS) analyses) were used to perform bioinformatic integration of GWAS data (N = 20,806, N = 59,804) with 'omics reference datasets including the blood (eQTLgen consortium N = 31,684) and brain (N = 2581). This was followed up by specific expression studies in ALS case-control cohorts (microarray N = 942, protein N = 300) and gene knockdown (KD) studies of human neuronal iPSC cells and zebrafish-morpholinos (MO).
RESULTS: SMR analyses implicated both TNIP1 and GPX3 (p < 1.15 × 10), but there was no simple SNP/expression relationship. Integrating multiple datasets using PoPS supported GPX3 but not TNIP1. In vivo expression analyses from blood in ALS cases identified that lower GPX3 expression correlated with a more progressed disease (ALS functional rating score, p = 5.5 × 10, adjusted R = 0.042, B = 27.4 ± 13.3 ng/ml/ALSFRS unit) with microarray and protein data suggesting lower expression with risk allele (recessive model p = 0.06, p = 0.02 respectively). Validation in vivo indicated gpx3 KD caused significant motor deficits in zebrafish-MO (mean difference vs. control ± 95% CI, vs. control, swim distance = 112 ± 28 mm, time = 1.29 ± 0.59 s, speed = 32.0 ± 2.53 mm/s, respectively, p for all < 0.0001), which were rescued with gpx3 expression, with no phenotype identified with tnip1 KD or gpx3 overexpression.
CONCLUSIONS: These results support GPX3 as a lead ALS risk gene in this locus, with more data needed to confirm/reject a role for TNIP1. This has implications for understanding disease mechanisms (GPX3 acts in the same pathway as SOD1, a well-established ALS-associated gene) and identifying new therapeutic approaches. Few previous examples of in-depth investigations of risk loci in ALS exist and a similar approach could be applied to investigate future expected GWAS findings.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
METHODS: The Functional Mapping and Annotation (FUMA) pipeline and five tools (conditional and joint analysis (GCTA-COJO), Stratified Linkage Disequilibrium Score Regression (S-LDSC), Polygenic Priority Scoring (PoPS), Summary-based Mendelian Randomisation (SMR-HEIDI) and transcriptome-wide association study (TWAS) analyses) were used to perform bioinformatic integration of GWAS data (N = 20,806, N = 59,804) with ‘omics reference datasets including the blood (eQTLgen consortium N = 31,684) and brain (N = 2581). This was followed up by specific expression studies in ALS case-control cohorts (microarray N = 942, protein N = 300) and gene knockdown (KD) studies of human neuronal iPSC cells and zebrafish-morpholinos (MO).
RESULTS: SMR analyses implicated both TNIP1 and GPX3 (p < 1.15 × 10), but there was no simple SNP/expression relationship. Integrating multiple datasets using PoPS supported GPX3 but not TNIP1. In vivo expression analyses from blood in ALS cases identified that lower GPX3 expression correlated with a more progressed disease (ALS functional rating score, p = 5.5 × 10, adjusted R = 0.042, B = 27.4 ± 13.3 ng/ml/ALSFRS unit) with microarray and protein data suggesting lower expression with risk allele (recessive model p = 0.06, p = 0.02 respectively). Validation in vivo indicated gpx3 KD caused significant motor deficits in zebrafish-MO (mean difference vs. control ± 95% CI, vs. control, swim distance = 112 ± 28 mm, time = 1.29 ± 0.59 s, speed = 32.0 ± 2.53 mm/s, respectively, p for all < 0.0001), which were rescued with gpx3 expression, with no phenotype identified with tnip1 KD or gpx3 overexpression.
CONCLUSIONS: These results support GPX3 as a lead ALS risk gene in this locus, with more data needed to confirm/reject a role for TNIP1. This has implications for understanding disease mechanisms (GPX3 acts in the same pathway as SOD1, a well-established ALS-associated gene) and identifying new therapeutic approaches. Few previous examples of in-depth investigations of risk loci in ALS exist and a similar approach could be applied to investigate future expected GWAS findings.
2021

Harriet P Lo, Ye-Wheen Lim, Zherui Xiong, Nick Martel, Charles Ferguson, Nicholas Ariotti, Jean Giacomotto, James Rae, Matthias Floetenmeyer, Shayli Varasteh Moradi, Ya Gao, Vikas A Tillu, Di Xia, Huang Wang, Samira Rahnama, Susan J Nixon, Michele Bastiani, Ryan D Day, Kelly A Smith, Nathan J Palpant, Wayne A Johnston, Kirill Alexandrov, Brett M Collins, Thomas E Hall, Robert G Parton
Cavin4 interacts with Bin1 to promote T-tubule formation and stability in developing skeletal muscle Journal Article
In: J Cell Biol, vol. 220, no. 12, 2021, ISSN: 1540-8140.
@article{pmid34633413,
title = {Cavin4 interacts with Bin1 to promote T-tubule formation and stability in developing skeletal muscle},
author = {Harriet P Lo and Ye-Wheen Lim and Zherui Xiong and Nick Martel and Charles Ferguson and Nicholas Ariotti and Jean Giacomotto and James Rae and Matthias Floetenmeyer and Shayli Varasteh Moradi and Ya Gao and Vikas A Tillu and Di Xia and Huang Wang and Samira Rahnama and Susan J Nixon and Michele Bastiani and Ryan D Day and Kelly A Smith and Nathan J Palpant and Wayne A Johnston and Kirill Alexandrov and Brett M Collins and Thomas E Hall and Robert G Parton},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/jcb_201905065.pdf},
doi = {10.1083/jcb.201905065},
issn = {1540-8140},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {J Cell Biol},
volume = {220},
number = {12},
abstract = {The cavin proteins are essential for caveola biogenesis and function. Here, we identify a role for the muscle-specific component, Cavin4, in skeletal muscle T-tubule development by analyzing two vertebrate systems, mouse and zebrafish. In both models, Cavin4 localized to T-tubules, and loss of Cavin4 resulted in aberrant T-tubule maturation. In zebrafish, which possess duplicated cavin4 paralogs, Cavin4b was shown to directly interact with the T-tubule-associated BAR domain protein Bin1. Loss of both Cavin4a and Cavin4b caused aberrant accumulation of interconnected caveolae within the T-tubules, a fragmented T-tubule network enriched in Caveolin-3, and an impaired Ca2+ response upon mechanical stimulation. We propose a role for Cavin4 in remodeling the T-tubule membrane early in development by recycling caveolar components from the T-tubule to the sarcolemma. This generates a stable T-tubule domain lacking caveolae that is essential for T-tubule function.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Alisha Tromp, Bryan Mowry, Jean Giacomotto
Neurexins in autism and schizophrenia-a review of patient mutations, mouse models and potential future directions Journal Article
In: Mol Psychiatry, vol. 26, no. 3, pp. 747–760, 2021, ISSN: 1476-5578.
@article{pmid33191396,
title = {Neurexins in autism and schizophrenia-a review of patient mutations, mouse models and potential future directions},
author = {Alisha Tromp and Bryan Mowry and Jean Giacomotto},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/Neurexins-in-autism-and-schizophrenia-6.pdf},
doi = {10.1038/s41380-020-00944-8},
issn = {1476-5578},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Mol Psychiatry},
volume = {26},
number = {3},
pages = {747--760},
abstract = {Mutations in the family of neurexins (NRXN1, NRXN2 and NRXN3) have been repeatedly identified in patients with autism spectrum disorder (ASD) and schizophrenia (SCZ). However, it remains unclear how these DNA variants affect neurexin functions and thereby predispose to these neurodevelopmental disorders. Understanding both the wild-type and pathologic roles of these genes in the brain could help unveil biological mechanisms underlying mental disorders. In this regard, numerous studies have focused on generating relevant loss-of-function (LOF) mammalian models. Although this has increased our knowledge about their normal functions, the potential pathologic role(s) of these human variants remains elusive. Indeed, after reviewing the literature, it seems apparent that a traditional LOF-genetic approach based on complete LOF might not be sufficient to unveil the role of these human mutations. First, these genes present a very complex transcriptome and total-LOF of all isoforms may not be the cause of toxicity in patients, particularly given evidence that causative variants act through haploinsufficiency. Moreover, human DNA variants may not all lead to LOF but potentially to intricate transcriptome changes that could also include the generation of aberrant isoforms acting as a gain-of-function (GOF). Furthermore, their transcriptomic complexity most likely renders them prone to genetic compensation when one tries to manipulate them using traditional site-directed mutagenesis approaches, and this could act differently from model to model leading to heterogeneous and conflicting phenotypes. This review compiles the relevant literature on variants identified in human studies and on the mouse models currently deployed, and offers suggestions for future research.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Alisha Tromp, Kate Robinson, Thomas E Hall, Bryan Mowry, Jean Giacomotto
Pipeline for generating stable large genomic deletions in zebrafish, from small domains to whole gene excisions Journal Article
In: G3 (Bethesda), vol. 11, no. 12, 2021, ISSN: 2160-1836.
@article{pmid34499171,
title = {Pipeline for generating stable large genomic deletions in zebrafish, from small domains to whole gene excisions},
author = {Alisha Tromp and Kate Robinson and Thomas E Hall and Bryan Mowry and Jean Giacomotto},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/OP-GTHE210325-1..6-1.pdf},
doi = {10.1093/g3journal/jkab321},
issn = {2160-1836},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {G3 (Bethesda)},
volume = {11},
number = {12},
abstract = {Here we describe a short feasibility study and methodological framework for the production of stable, CRISPR/Cas9-based, large genomic deletions in zebrafish, ranging from several base pairs (bp) to hundreds of kilobases (kb). Using a cocktail of four single guide RNAs (sgRNAs) targeting a single genomic region mixed with a marker-sgRNA against the pigmentation gene tyrosinase, we demonstrate that one can easily and accurately excise genomic regions such as promoters, protein domains, specific exons, or whole genes. We exemplify this technique with a complex gene family, neurexins, composed of three duplicated genes with multiple promoters and intricate splicing processes leading to thousands of isoforms. We precisely deleted small regions such as their transmembrane domains (150 bp deletion in average) to their entire genomic locus (300 kb deletion for nrxn1a for instance). We find that both the concentration and ratio of Cas9/sgRNAs are critical for the successful generation of these large deletions and, interestingly, that in our study, their transmission frequency does not seem to decrease with increasing distance between sgRNA target sites. Considering the growing reports and debate about genetically compensated small indel mutants, the use of large-deletion approaches is likely to be widely adopted in studies of gene function. This strategy will also be key to the study of non-coding genomic regions. Note that we are also describing here a custom method to produce the sgRNAs, which proved to be faster and more robust than the ones traditionally used in the community to date.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Zoé Butti, Yingzhou Edward Pan, Jean Giacomotto, Shunmoogum A Patten
Reduced C9orf72 function leads to defective synaptic vesicle release and neuromuscular dysfunction in zebrafish Journal Article
In: Commun Biol, vol. 4, no. 1, pp. 792, 2021, ISSN: 2399-3642.
@article{pmid34172817,
title = {Reduced C9orf72 function leads to defective synaptic vesicle release and neuromuscular dysfunction in zebrafish},
author = {Zoé Butti and Yingzhou Edward Pan and Jean Giacomotto and Shunmoogum A Patten},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/Reduced-C9orf72-function-leads-to-defective-synaptic-vesicle-release-and-neuromuscular-dysfunction-in-zebrafish.pdf},
doi = {10.1038/s42003-021-02302-y},
issn = {2399-3642},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Commun Biol},
volume = {4},
number = {1},
pages = {792},
abstract = {The most common genetic cause of amyotrophic lateral sclerosis (ALS) and fronto-temporal dementia (FTD) is a hexanucleotide repeat expansion within the C9orf72 gene. Reduced levels of C9orf72 mRNA and protein have been found in ALS/FTD patients, but the role of this protein in disease pathogenesis is still poorly understood. Here, we report the generation and characterization of a stable C9orf72 loss-of-function (LOF) model in the zebrafish. We show that reduced C9orf72 function leads to motor defects, muscle atrophy, motor neuron loss and mortality in early larval and adult stages. Analysis of the structure and function of the neuromuscular junctions (NMJs) of the larvae, reveal a marked reduction in the number of presynaptic and postsynaptic structures and an impaired release of quantal synaptic vesicles at the NMJ. Strikingly, we demonstrate a downregulation of SV2a upon C9orf72-LOF and a reduced rate of synaptic vesicle cycling. Furthermore, we show a reduced number and size of Rab3a-postive synaptic puncta at NMJs. Altogether, these results reveal a key function for C9orf72 in the control of presynaptic vesicle trafficking and release at the zebrafish larval NMJ. Our study demonstrates an important role for C9orf72 in ALS/FTD pathogenesis, where it regulates synaptic vesicle release and neuromuscular functions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2020
Rachel S Gormal, Pranesh Padmanabhan, Ravikiran Kasula, Adekunle T Bademosi, Sean Coakley, Jean Giacomotto, Ailisa Blum, Merja Joensuu, Tristan P Wallis, Harriet P Lo, Srikanth Budnar, James Rae, Charles Ferguson, Michele Bastiani, Walter G Thomas, Els Pardon, Jan Steyaert, Alpha S Yap, Geoffrey J Goodhill, Massimo A Hilliard, Robert G Parton, Frédéric A Meunier
Modular transient nanoclustering of activated β2-adrenergic receptors revealed by single-molecule tracking of conformation-specific nanobodies Journal Article
In: Proc Natl Acad Sci U S A, vol. 117, no. 48, pp. 30476–30487, 2020, ISSN: 1091-6490.
@article{pmid33214152,
title = {Modular transient nanoclustering of activated β2-adrenergic receptors revealed by single-molecule tracking of conformation-specific nanobodies},
author = {Rachel S Gormal and Pranesh Padmanabhan and Ravikiran Kasula and Adekunle T Bademosi and Sean Coakley and Jean Giacomotto and Ailisa Blum and Merja Joensuu and Tristan P Wallis and Harriet P Lo and Srikanth Budnar and James Rae and Charles Ferguson and Michele Bastiani and Walter G Thomas and Els Pardon and Jan Steyaert and Alpha S Yap and Geoffrey J Goodhill and Massimo A Hilliard and Robert G Parton and Frédéric A Meunier},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/Modular-transient-nanoclustering-of-activated-β2-adrenergic-receptors-revealed-by-single-molecule-tracking-of-conformation-specific-nanobodies.pdf},
doi = {10.1073/pnas.2007443117},
issn = {1091-6490},
year = {2020},
date = {2020-01-01},
urldate = {2020-01-01},
journal = {Proc Natl Acad Sci U S A},
volume = {117},
number = {48},
pages = {30476--30487},
abstract = {None of the current superresolution microscopy techniques can reliably image the changes in endogenous protein nanoclustering dynamics associated with specific conformations in live cells. Single-domain nanobodies have been invaluable tools to isolate defined conformational states of proteins, and we reasoned that expressing these nanobodies coupled to single-molecule imaging-amenable tags could allow superresolution analysis of endogenous proteins in discrete conformational states. Here, we used anti-GFP nanobodies tagged with photoconvertible mEos expressed as intrabodies, as a proof-of-concept to perform single-particle tracking on a range of GFP proteins expressed in live cells, neurons, and small organisms. We next expressed highly specialized nanobodies that target conformation-specific endogenous β-adrenoreceptor (β-AR) in neurosecretory cells, unveiling real-time mobility behaviors of activated and inactivated endogenous conformers during agonist treatment in living cells. We showed that activated β- (Nb80) is highly immobile and organized in nanoclusters. The Gαs-GPCR complex detected with Nb37 displayed higher mobility with surprisingly similar nanoclustering dynamics to that of Nb80. Activated conformers are highly sensitive to dynamin inhibition, suggesting selective targeting for endocytosis. Inactivated β- (Nb60) molecules are also largely immobile but relatively less sensitive to endocytic blockade. Expression of single-domain nanobodies therefore provides a unique opportunity to capture highly transient changes in the dynamic nanoscale organization of endogenous proteins.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Dan Wang, S W A Himaya, Jean Giacomotto, Md Mahadhi Hasan, Fernanda C Cardoso, Lotten Ragnarsson, Richard J Lewis
Characterisation of d-Conotoxin TxVIA as a Mammalian T-Type Calcium Channel Modulator Journal Article
In: Mar Drugs, vol. 18, no. 7, 2020, ISSN: 1660-3397.
@article{pmid32629781,
title = {Characterisation of d-Conotoxin TxVIA as a Mammalian T-Type Calcium Channel Modulator},
author = {Dan Wang and S W A Himaya and Jean Giacomotto and Md Mahadhi Hasan and Fernanda C Cardoso and Lotten Ragnarsson and Richard J Lewis},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/acs.jproteome.5b00630.pdf},
doi = {10.3390/md18070343},
issn = {1660-3397},
year = {2020},
date = {2020-06-01},
urldate = {2020-06-01},
journal = {Mar Drugs},
volume = {18},
number = {7},
abstract = {The 27-amino acid (aa)-long d-conotoxin TxVIA, originally isolated from the mollusc-hunting cone snail , slows voltage-gated sodium (Na) channel inactivation in molluscan neurons, but its mammalian ion channel targets remain undetermined. In this study, we confirmed that TxVIA was inactive on mammalian Na1.2 and Na1.7 even at high concentrations (10 µM). Given the fact that invertebrate Na channel and T-type calcium channels (Ca3.x) are evolutionarily related, we examined the possibility that TxVIA may act on Ca3.x. Electrophysiological characterisation of the native TxVIA on Ca3.1, 3.2 and 3.3 revealed that TxVIA preferentially inhibits Ca3.2 current (IC = 0.24 mM) and enhances Ca3.1 current at higher concentrations. In fish bioassays TxVIA showed little effect on zebrafish behaviours when injected intramuscular at 250 ng/100 mg fish. The binding sites for TxVIA at Na1.7 and Ca3.1 revealed that their channel binding sites contained a common epitope.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2019

Sathish Periyasamy, Sujit John, Raman Padmavati, Preeti Rajendren, Priyadarshini Thirunavukkarasu, Jacob Gratten, Anna Vinkhuyzen, Allan McRae, Elizabeth G Holliday, Dale R Nyholt, Derek Nancarrow, Andrew Bakshi, Gibran Hemani, Deborah Nertney, Heather Smith, Cheryl Filippich, Kalpana Patel, Javed Fowdar, Duncan McLean, Srinivasan Tirupati, Arunkumar Nagasundaram, Prasad Rao Gundugurti, Krishnamurthy Selvaraj, Jayaprakash Jegadeesan, Lynn B Jorde, Naomi R Wray, Matthew A Brown, Rachel Suetani, Jean Giacomotto, Rangaswamy Thara, Bryan J Mowry
Association of Schizophrenia Risk With Disordered Niacin Metabolism in an Indian Genome-wide Association Study Journal Article
In: JAMA Psychiatry, vol. 76, no. 10, pp. 1026–1034, 2019, ISSN: 2168-6238.
@article{pmid31268507,
title = {Association of Schizophrenia Risk With Disordered Niacin Metabolism in an Indian Genome-wide Association Study},
author = {Sathish Periyasamy and Sujit John and Raman Padmavati and Preeti Rajendren and Priyadarshini Thirunavukkarasu and Jacob Gratten and Anna Vinkhuyzen and Allan McRae and Elizabeth G Holliday and Dale R Nyholt and Derek Nancarrow and Andrew Bakshi and Gibran Hemani and Deborah Nertney and Heather Smith and Cheryl Filippich and Kalpana Patel and Javed Fowdar and Duncan McLean and Srinivasan Tirupati and Arunkumar Nagasundaram and Prasad Rao Gundugurti and Krishnamurthy Selvaraj and Jayaprakash Jegadeesan and Lynn B Jorde and Naomi R Wray and Matthew A Brown and Rachel Suetani and Jean Giacomotto and Rangaswamy Thara and Bryan J Mowry},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/jamapsychiatry_periyasamy_2019_oi_190035.pdf},
doi = {10.1001/jamapsychiatry.2019.1335},
issn = {2168-6238},
year = {2019},
date = {2019-01-01},
urldate = {2019-01-01},
journal = {JAMA Psychiatry},
volume = {76},
number = {10},
pages = {1026--1034},
abstract = {Importance: Genome-wide association studies (GWASs) in European populations have identified more than 100 schizophrenia-associated loci. A schizophrenia GWAS in a unique Indian population offers novel findings.
Objective: To discover and functionally evaluate genetic loci for schizophrenia in a GWAS of a unique Indian population.
Design, Setting, and Participants: This GWAS included a sample of affected individuals, family members, and unrelated cases and controls. Three thousand ninety-two individuals were recruited and diagnostically ascertained via medical records, hospitals, clinics, and clinical networks in Chennai and surrounding regions. Affected participants fulfilled DSM-IV diagnostic criteria for schizophrenia. Unrelated control participants had no personal or family history of psychotic disorder. Recruitment, genotyping, and analysis occurred in consecutive phases beginning January 1, 2001. Recruitment was completed on February 28, 2018, and genotyping and analysis are ongoing.
Main Outcomes and Measures: Associations of single-nucleotide polymorphisms and gene expression with schizophrenia.
Results: The study population included 1321 participants with schizophrenia, 885 family controls, and 886 unrelated controls. Among participants with schizophrenia, mean (SD) age was 39.1 (11.4) years, and 52.7% were male. This sample demonstrated uniform ethnicity, a degree of inbreeding, and negligible rates of substance abuse. A novel genome-wide significant association was observed between schizophrenia and a chromosome 8q24.3 locus (rs10866912, allele A; odds ratio [OR], 1.27 [95% CI, 1.17-1.38]; P = 4.35 × 10-8) that attracted support in the schizophrenia Psychiatric Genomics Consortium 2 data (rs10866912, allele A; OR, 1.04 [95% CI, 1.02-1.06]; P = 7.56 × 10-4). This locus has undergone natural selection, with the risk allele A declining in frequency from India (approximately 72%) to Europe (approximately 43%). rs10866912 directly modifies the abundance of the nicotinate phosphoribosyltransferase gene (NAPRT1) transcript in brain cortex (normalized effect size, 0.79; 95% CI, 0.6-1.0; P = 5.8 × 10-13). NAPRT1 encodes a key enzyme for niacin metabolism. In Indian lymphoblastoid cell lines, (risk) allele A of rs10866912 was associated with NAPRT1 downregulation (AA: 0.74, n = 21; CC: 1.56, n = 17; P = .004). Preliminary zebrafish data further suggest that partial loss of function of NAPRT1 leads to abnormal brain development.
Conclusions and Relevance: Bioinformatic analyses and cellular and zebrafish gene expression studies implicate NAPRT1 as a novel susceptibility gene. Given this gene's role in niacin metabolism and the evidence for niacin deficiency provoking schizophrenialike symptoms in neuropsychiatric diseases such as pellagra and Hartnup disease, these results suggest that the rs10866912 genotype and niacin status may have implications for schizophrenia susceptibility and treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Objective: To discover and functionally evaluate genetic loci for schizophrenia in a GWAS of a unique Indian population.
Design, Setting, and Participants: This GWAS included a sample of affected individuals, family members, and unrelated cases and controls. Three thousand ninety-two individuals were recruited and diagnostically ascertained via medical records, hospitals, clinics, and clinical networks in Chennai and surrounding regions. Affected participants fulfilled DSM-IV diagnostic criteria for schizophrenia. Unrelated control participants had no personal or family history of psychotic disorder. Recruitment, genotyping, and analysis occurred in consecutive phases beginning January 1, 2001. Recruitment was completed on February 28, 2018, and genotyping and analysis are ongoing.
Main Outcomes and Measures: Associations of single-nucleotide polymorphisms and gene expression with schizophrenia.
Results: The study population included 1321 participants with schizophrenia, 885 family controls, and 886 unrelated controls. Among participants with schizophrenia, mean (SD) age was 39.1 (11.4) years, and 52.7% were male. This sample demonstrated uniform ethnicity, a degree of inbreeding, and negligible rates of substance abuse. A novel genome-wide significant association was observed between schizophrenia and a chromosome 8q24.3 locus (rs10866912, allele A; odds ratio [OR], 1.27 [95% CI, 1.17-1.38]; P = 4.35 × 10-8) that attracted support in the schizophrenia Psychiatric Genomics Consortium 2 data (rs10866912, allele A; OR, 1.04 [95% CI, 1.02-1.06]; P = 7.56 × 10-4). This locus has undergone natural selection, with the risk allele A declining in frequency from India (approximately 72%) to Europe (approximately 43%). rs10866912 directly modifies the abundance of the nicotinate phosphoribosyltransferase gene (NAPRT1) transcript in brain cortex (normalized effect size, 0.79; 95% CI, 0.6-1.0; P = 5.8 × 10-13). NAPRT1 encodes a key enzyme for niacin metabolism. In Indian lymphoblastoid cell lines, (risk) allele A of rs10866912 was associated with NAPRT1 downregulation (AA: 0.74, n = 21; CC: 1.56, n = 17; P = .004). Preliminary zebrafish data further suggest that partial loss of function of NAPRT1 leads to abnormal brain development.
Conclusions and Relevance: Bioinformatic analyses and cellular and zebrafish gene expression studies implicate NAPRT1 as a novel susceptibility gene. Given this gene’s role in niacin metabolism and the evidence for niacin deficiency provoking schizophrenialike symptoms in neuropsychiatric diseases such as pellagra and Hartnup disease, these results suggest that the rs10866912 genotype and niacin status may have implications for schizophrenia susceptibility and treatment.
Mriga Dutt, Jean Giacomotto, Lotten Ragnarsson, Åsa Andersson, Andreas Brust, Zoltan Dekan, Paul F Alewood, Richard J Lewis
The α-adrenoceptor inhibitor ρ-TIA facilitates net hunting in piscivorous Conus tulipa Journal Article
In: Sci Rep, vol. 9, no. 1, pp. 17841, 2019, ISSN: 2045-2322.
@article{pmid31780714,
title = {The α-adrenoceptor inhibitor ρ-TIA facilitates net hunting in piscivorous Conus tulipa},
author = {Mriga Dutt and Jean Giacomotto and Lotten Ragnarsson and Åsa Andersson and Andreas Brust and Zoltan Dekan and Paul F Alewood and Richard J Lewis},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/s41598-019-54186-y.pdf},
doi = {10.1038/s41598-019-54186-y},
issn = {2045-2322},
year = {2019},
date = {2019-01-01},
urldate = {2019-01-01},
journal = {Sci Rep},
volume = {9},
number = {1},
pages = {17841},
abstract = {Cone snails use separately evolved venoms for prey capture and defence. While most use a harpoon for prey capture, the Gastridium clade that includes the well-studied Conus geographus and Conus tulipa, have developed a net hunting strategy to catch fish. This unique feeding behaviour requires secretion of "nirvana cabal" peptides to dampen the escape response of targeted fish allowing for their capture directly by mouth. However, the active components of the nirvana cabal remain poorly defined. In this study, we evaluated the behavioural effects of likely nirvana cabal peptides on the teleost model, Danio rerio (zebrafish). Surprisingly, the conantokins (NMDA receptor antagonists) and/or conopressins (vasopressin receptor agonists and antagonists) found in C. geographus and C. tulipa venom failed to produce a nirvana cabal-like effect in zebrafish. In contrast, low concentrations of the non-competitive adrenoceptor antagonist ρ-TIA found in C. tulipa venom (EC = 190 nM) dramatically reduced the escape response of zebrafish larvae when added directly to aquarium water. ρ-TIA inhibited the zebrafish α-adrenoceptor, confirming ρ-TIA has the potential to reverse the known stimulating effects of norepinephrine on fish behaviour. ρ-TIA may act alone and not as part of a cabal, since it did not synergise with conopressins and/or conantokins. This study highlights the importance of using ecologically relevant animal behaviour models to decipher the complex neurobiology underlying the prey capture and defensive strategies of cone snails.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2017

Ye-Wheen Lim, Harriet P Lo, Charles Ferguson, Nick Martel, Jean Giacomotto, Guillermo A Gomez, Alpha S Yap, Thomas E Hall, Robert G Parton
Caveolae Protect Notochord Cells against Catastrophic Mechanical Failure during Development Journal Article
In: Curr Biol, vol. 27, no. 13, pp. 1968–1981.e7, 2017, ISSN: 1879-0445.
@article{pmid28648821,
title = {Caveolae Protect Notochord Cells against Catastrophic Mechanical Failure during Development},
author = {Ye-Wheen Lim and Harriet P Lo and Charles Ferguson and Nick Martel and Jean Giacomotto and Guillermo A Gomez and Alpha S Yap and Thomas E Hall and Robert G Parton},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/PIIS0960982217306334-1.pdf},
doi = {10.1016/j.cub.2017.05.067},
issn = {1879-0445},
year = {2017},
date = {2017-07-01},
urldate = {2017-07-01},
journal = {Curr Biol},
volume = {27},
number = {13},
pages = {1968--1981.e7},
abstract = {The embryonic notochord is a flexible structure present during development that serves as scaffold for formation of the vertebrate spine. This rod-like organ is thought to have evolved in non-vertebrate chordates to facilitate locomotion by providing a rigid but flexible midline structure against which the axial muscles can contract. This hydrostatic "skeleton" is exposed to a variety of mechanical forces during oscillation of the body. There is evidence that caveolae, submicroscopic cup-shaped plasma membrane pits, can buffer tension in cells that undergo high levels of mechanical stress. Indeed, caveolae are particularly abundant in the embryonic notochord. In this study, we used the CRISPR/Cas9 system to generate a mutant zebrafish line lacking Cavin1b, a coat protein required for caveola formation. Our cavin1b zebrafish line exhibits reduced locomotor capacity and prominent notochord lesions characterized by necrotic, damaged, and membrane-permeable cells. Notochord diameter and body length are reduced, but remarkably, the mutants recover and are homozygous viable. By manipulating mechanical stress using a number of different assays, we show that progression of lesion severity in the mutant notochord is directly dependent on locomotion. We also demonstrate changes in caveola morphology in vivo in response to mechanical stress. Finally, induction of a catastrophic collapse of live cavin1b mutant notochord cells provides the first real-time observation of caveolae mediating cellular mechanoprotection.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2016

J Giacomotto, A P Carroll, S Rinkwitz, B Mowry, M J Cairns, T S Becker
Developmental suppression of schizophrenia-associated miR-137 alters sensorimotor function in zebrafish Journal Article
In: Transl Psychiatry, vol. 6, pp. e818, 2016, ISSN: 2158-3188.
@article{pmid27219344,
title = {Developmental suppression of schizophrenia-associated miR-137 alters sensorimotor function in zebrafish},
author = {J Giacomotto and A P Carroll and S Rinkwitz and B Mowry and M J Cairns and T S Becker},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/tp201688a.pdf},
doi = {10.1038/tp.2016.88},
issn = {2158-3188},
year = {2016},
date = {2016-01-01},
urldate = {2016-01-01},
journal = {Transl Psychiatry},
volume = {6},
pages = {e818},
abstract = {The neurodevelopmentally regulated microRNA miR-137 was strongly implicated as risk locus for schizophrenia in the most recent genome wide association study coordinated by the Psychiatric Genome Consortium (PGC). This molecule is highly conserved in vertebrates enabling the investigation of its function in the developing zebrafish. We utilized this model system to achieve overexpression and suppression of miR-137, both transiently and stably through transgenesis. While miR-137 overexpression was not associated with an observable specific phenotype, downregulation by antisense morpholino and/or transgenic expression of miR-sponge RNA induced significant impairment of both embryonic and larval touch-sensitivity without compromising overall anatomical development. We observed miR-137 expression and activity in sensory neurons including Rohon-Beard neurons and dorsal root ganglia, two neuronal cell types that confer touch-sensitivity in normal zebrafish, suggesting a role of these cell types in the observed phenotype. The lack of obvious anatomical or histological pathology in these cells, however, suggested that subtle axonal network defects or a change in synaptic function and neural connectivity might be responsible for the behavioral phenotype rather than a change in the cellular morphology or neuroanatomy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Angela S Laird, Nikolce Mackovski, Silke Rinkwitz, Thomas S Becker, Jean Giacomotto
Tissue-specific models of spinal muscular atrophy confirm a critical role of SMN in motor neurons from embryonic to adult stages Journal Article
In: Hum Mol Genet, vol. 25, no. 9, pp. 1728–1738, 2016, ISSN: 1460-2083.
@article{pmid26908606,
title = {Tissue-specific models of spinal muscular atrophy confirm a critical role of SMN in motor neurons from embryonic to adult stages},
author = {Angela S Laird and Nikolce Mackovski and Silke Rinkwitz and Thomas S Becker and Jean Giacomotto},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/Hum.-Mol.-Genet.-2016-Laird-hmg-ddw044.pdf},
doi = {10.1093/hmg/ddw044},
issn = {1460-2083},
year = {2016},
date = {2016-01-01},
urldate = {2016-01-01},
journal = {Hum Mol Genet},
volume = {25},
number = {9},
pages = {1728--1738},
abstract = {Spinal muscular atrophy (SMA) is an autosomal recessive disease linked to survival motor neuron (SMN) protein deficiency. While SMN protein is expressed ubiquitously, its deficiency triggers tissue-specific hallmarks, including motor neuron death and muscle atrophy, leading to impaired motor functions and premature death. Here, using stable miR-mediated knockdown technology in zebrafish, we developed the first vertebrate system allowing transgenic spatio-temporal control of the smn1 gene. Using this new model it is now possible to investigate normal and pathogenic SMN function(s) in specific cell types, independently or in synergy with other cell populations. We took advantage of this new system to first test the effect of motor neuron or muscle-specific smn1 silencing. Anti-smn1 miRNA expression in motor neurons, but not in muscles, reproduced SMA hallmarks, including abnormal motor neuron development, poor motor function and premature death. Interestingly, smn1 knockdown in motor neurons also induced severe late-onset phenotypes including scoliosis-like body deformities, weight loss, muscle atrophy and, seen for the first time in zebrafish, reduction in the number of motor neurons, indicating motor neuron degeneration. Taken together, we have developed a new transgenic system allowing spatio-temporal control of smn1 expression in zebrafish, and using this model, we have demonstrated that smn1 silencing in motor neurons alone is sufficient to reproduce SMA hallmarks in zebrafish. It is noteworthy that this research is going beyond SMA as this versatile gene-silencing transgenic system can be used to knockdown any genes of interest, filling the gap in the zebrafish genetic toolbox and opening new avenues to study gene functions in this organism.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Adam J Svahn, Jean Giacomotto, Manuel B Graeber, Silke Rinkwitz, Thomas S Becker
miR-124 Contributes to the functional maturity of microglia Journal Article
In: Dev Neurobiol, vol. 76, no. 5, pp. 507–518, 2016, ISSN: 1932-846X.
@article{pmid26184457,
title = {miR-124 Contributes to the functional maturity of microglia},
author = {Adam J Svahn and Jean Giacomotto and Manuel B Graeber and Silke Rinkwitz and Thomas S Becker},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/Dev-Neurobiol.-miR-124.pdf},
doi = {10.1002/dneu.22328},
issn = {1932-846X},
year = {2016},
date = {2016-05-01},
urldate = {2016-05-01},
journal = {Dev Neurobiol},
volume = {76},
number = {5},
pages = {507--518},
abstract = {During early development of the central nervous system (CNS), a subset of yolk-sac derived myeloid cells populate the brain and provide the seed for the microglial cell population, which will self-renew throughout life. As development progresses, individual microglial cells transition from a phagocytic amoeboid state through a transitional morphing phase into the sessile, ramified, and normally nonphagocytic microglia observed in the adult CNS under healthy conditions. The molecular drivers of this tissue-specific maturation profile are not known. However, a survey of tissue resident macrophages identified miR-124 to be expressed in microglia. In this study, we used transgenic zebrafish to overexpress miR-124 in the mpeg1 expressing yolk-sac-derived myeloid cells that seed the microglia. In addition, a systemic sponge designed to neutralize the effects of miR-124 was used to assess microglial development in a miR-124 loss-of-function environment. Following the induction of miR-124 overexpression, microglial motility and phagocytosis of apoptotic cells were significantly reduced. miR-124 overexpression in microglia resulted in the accumulation of residual apoptotic cell bodies in the optic tectum, which could not be achieved by miR-124 overexpression in differentiated neurons. Conversely, expression of the miR-124 sponge caused an increase in the motility of microglia and transiently rescued motility and phagocytosis functions when activated simultaneously with miR-124 overexpression. This study provides in vivo evidence that miR-124 activity has a key role in the development of functionally mature microglia.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2015
Beena Punnamoottil, Silke Rinkwitz, Jean Giacomotto, Adam J Svahn, Thomas S Becker
Motor neuron-expressed microRNAs 218 and their enhancers are nested within introns of Slit2/3 genes Journal Article
In: Genesis, vol. 53, no. 5, pp. 321–328, 2015, ISSN: 1526-968X.
@article{pmid25864959,
title = {Motor neuron-expressed microRNAs 218 and their enhancers are nested within introns of Slit2/3 genes},
author = {Beena Punnamoottil and Silke Rinkwitz and Jean Giacomotto and Adam J Svahn and Thomas S Becker},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/Genesis-2015-Punnamoottil.pdf},
doi = {10.1002/dvg.22852},
issn = {1526-968X},
year = {2015},
date = {2015-05-01},
urldate = {2015-05-01},
journal = {Genesis},
volume = {53},
number = {5},
pages = {321--328},
abstract = {miR218-1 and miR218-2 are embedded in introns of SLIT2 and SLIT3, respectively, an arrangement conserved throughout vertebrate genomes. Both miR218 genes are predicted to be transcribed in the same orientation as their host genes and were assumed to be spliced from Slit2/3 primary transcripts. In zebrafish miR218 is active in cranial nerve motor nuclei and spinal cord motor neurons, while slit2 and slit3 are expressed predominantly in the midline. This differential expression pattern suggested independent regulation of miR218 genes by distinct enhancers. We tested conserved noncoding elements for regulatory activity by reporter gene transgenesis in zebrafish. Two human enhancers, 76 kb and 130 kb distant from miR218-2, were identified that drove GFP expression in zebrafish in an almost complete miR218 expression pattern. In the zebrafish slit3 locus, two enhancers with identical activity were discovered. In human SLIT2 one enhancer 52 kb upstream of miR218-1 drove an expression pattern very similar to the enhancers of miR218-2. This establishes that miR218-1/-2 regulatory units are nested within SLIT2/3 and that they are duplicates of an ancestral single locus. Due to the strong activity of the enhancers, unique transgenic lines were created that facilitate morphological and gene functional genetic experiments in motor neurons.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Jean Giacomotto, Silke Rinkwitz, Thomas S Becker
Effective heritable gene knockdown in zebrafish using synthetic microRNAs Journal Article
In: Nat Commun, vol. 6, pp. 7378, 2015, ISSN: 2041-1723.
@article{pmid26051838,
title = {Effective heritable gene knockdown in zebrafish using synthetic microRNAs},
author = {Jean Giacomotto and Silke Rinkwitz and Thomas S Becker},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2015-Nat-comms-SMA.pdf},
doi = {10.1038/ncomms8378},
issn = {2041-1723},
year = {2015},
date = {2015-06-01},
urldate = {2015-06-01},
journal = {Nat Commun},
volume = {6},
pages = {7378},
abstract = {Although zebrafish is used to model human diseases through mutational and morpholino-based knockdown approaches, there are currently no robust transgenic knockdown tools. Here we investigate the knockdown efficiency of three synthetic miRNA-expressing backbones and show that these constructs can downregulate a sensor transgene with different degrees of potency. Using this approach, we reproduce spinal muscular atrophy (SMA) in zebrafish by targeting the smn1 gene. We also generate different transgenic lines, with severity and age of onset correlated to the level of smn1 inhibition, recapitulating for the first time the different forms of SMA in zebrafish. These lines are proof-of-concept that miRNA-based approaches can be used to generate potent heritable gene knockdown in zebrafish. },
keywords = {},
pubstate = {published},
tppubtype = {article}
}

S W A Himaya, Ai-Hua Jin, Sébastien Dutertre, Jean Giacomotto, Hoshyar Mohialdeen, Irina Vetter, Paul F Alewood, Richard J Lewis
Comparative Venomics Reveals the Complex Prey Capture Strategy of the Piscivorous Cone Snail Conus catus Journal Article
In: J Proteome Res, vol. 14, no. 10, pp. 4372–4381, 2015, ISSN: 1535-3907.
@article{pmid26322961,
title = {Comparative Venomics Reveals the Complex Prey Capture Strategy of the Piscivorous Cone Snail Conus catus},
author = {S W A Himaya and Ai-Hua Jin and Sébastien Dutertre and Jean Giacomotto and Hoshyar Mohialdeen and Irina Vetter and Paul F Alewood and Richard J Lewis},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2015-Conesnails-venomics-1.pdf},
doi = {10.1021/acs.jproteome.5b00630},
issn = {1535-3907},
year = {2015},
date = {2015-10-01},
urldate = {2015-10-01},
journal = {J Proteome Res},
volume = {14},
number = {10},
pages = {4372--4381},
abstract = {Venomous marine cone snails produce a unique and remarkably diverse range of venom peptides (conotoxins and conopeptides) that have proven to be invaluable as pharmacological probes and leads to new therapies. Conus catus is a hook-and-line fish hunter from clade I, with ∼20 conotoxins identified, including the analgesic ω-conotoxin CVID (AM336). The current study unravels the venom composition of C. catus with tandem mass spectrometry and 454 sequencing data. From the venom gland transcriptome, 104 precursors were recovered from 11 superfamilies, with superfamily A (especially κA-) conotoxins dominating (77%) their venom. Proteomic analysis confirmed that κA-conotoxins dominated the predation-evoked milked venom of each of six C. catus analyzed and revealed remarkable intraspecific variation in both the intensity and type of conotoxins. High-throughput FLIPR assays revealed that the predation-evoked venom contained a range of conotoxins targeting the nAChR, Cav, and Nav ion channels, consistent with α- and ω-conotoxins being used for predation by C. catus. However, the κA-conotoxins did not act at these targets but induced potent and rapid immobilization followed by bursts of activity and finally paralysis when injected intramuscularly in zebrafish. Our venomics approach revealed the complexity of the envenomation strategy used by C. catus, which contains a mix of both excitatory and inhibitory venom peptides. },
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Minaka Ishibashi, Elizabeth Manning, Cheryl Shoubridge, Monika Krecsmarik, Thomas A Hawkins, Jean Giacomotto, Ting Zhao, Thomas Mueller, Patricia I Bader, Sau W Cheung, Pawel Stankiewicz, Nicole L Bain, Anna Hackett, Chilamakuri C S Reddy, Alejandro S Mechaly, Bernard Peers, Stephen W Wilson, Boris Lenhard, Laure Bally-Cuif, Jozef Gecz, Thomas S Becker, Silke Rinkwitz
Copy number variants in patients with intellectual disability affect the regulation of ARX transcription factor gene Journal Article
In: Hum Genet, vol. 134, no. 11-12, pp. 1163–1182, 2015, ISSN: 1432-1203.
@article{pmid26337422,
title = {Copy number variants in patients with intellectual disability affect the regulation of ARX transcription factor gene},
author = {Minaka Ishibashi and Elizabeth Manning and Cheryl Shoubridge and Monika Krecsmarik and Thomas A Hawkins and Jean Giacomotto and Ting Zhao and Thomas Mueller and Patricia I Bader and Sau W Cheung and Pawel Stankiewicz and Nicole L Bain and Anna Hackett and Chilamakuri C S Reddy and Alejandro S Mechaly and Bernard Peers and Stephen W Wilson and Boris Lenhard and Laure Bally-Cuif and Jozef Gecz and Thomas S Becker and Silke Rinkwitz},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/ARX.pdf},
doi = {10.1007/s00439-015-1594-x},
issn = {1432-1203},
year = {2015},
date = {2015-11-01},
urldate = {2015-11-01},
journal = {Hum Genet},
volume = {134},
number = {11-12},
pages = {1163--1182},
abstract = {Protein-coding mutations in the transcription factor-encoding gene ARX cause various forms of intellectual disability (ID) and epilepsy. In contrast, variations in surrounding non-coding sequences are correlated with milder forms of non-syndromic ID and autism and had suggested the importance of ARX gene regulation in the etiology of these disorders. We compile data on several novel and some already identified patients with or without ID that carry duplications of ARX genomic region and consider likely genetic mechanisms underlying the neurodevelopmental defects. We establish the long-range regulatory domain of ARX and identify its brain region-specific autoregulation. We conclude that neurodevelopmental disturbances in the patients may not simply arise from increased dosage due to ARX duplication. This is further exemplified by a small duplication involving a non-functional ARX copy, but with duplicated enhancers. ARX enhancers are located within a 504-kb region and regulate expression specifically in the forebrain in developing and adult zebrafish. Transgenic enhancer-reporter lines were used as in vivo tools to delineate a brain region-specific negative and positive autoregulation of ARX. We find autorepression of ARX in the telencephalon and autoactivation in the ventral thalamus. Fluorescently labeled brain regions in the transgenic lines facilitated the identification of neuronal outgrowth and pathfinding disturbances in the ventral thalamus and telencephalon that occur when arxa dosage is diminished. In summary, we have established a model for how breakpoints in long-range gene regulation alter the expression levels of a target gene brain region-specifically, and how this can cause subtle neuronal phenotypes relating to the etiology of associated neuropsychiatric disease. },
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2013
Jean Giacomotto, Nicolas Brouilly, Ludivine Walter, Marie-Christine Mariol, Joachim Berger, Laurent Ségalat, Thomas S Becker, Peter D Currie, Kathrin Gieseler
Chemical genetics unveils a key role of mitochondrial dynamics, cytochrome c release and IP3R activity in muscular dystrophy Journal Article
In: Hum Mol Genet, vol. 22, no. 22, pp. 4562–4578, 2013, ISSN: 1460-2083.
@article{pmid23804750,
title = {Chemical genetics unveils a key role of mitochondrial dynamics, cytochrome c release and IP3R activity in muscular dystrophy},
author = {Jean Giacomotto and Nicolas Brouilly and Ludivine Walter and Marie-Christine Mariol and Joachim Berger and Laurent Ségalat and Thomas S Becker and Peter D Currie and Kathrin Gieseler},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2013-Chemical-genetics-unveils-a-key-role-of-mitochondrial-dynamics-cytochrome-c-release-and-IP3R-activity-in-muscular-dystrophy.pdf},
doi = {10.1093/hmg/ddt302},
issn = {1460-2083},
year = {2013},
date = {2013-11-01},
urldate = {2013-11-01},
journal = {Hum Mol Genet},
volume = {22},
number = {22},
pages = {4562--4578},
abstract = {Duchenne muscular dystrophy (DMD) is a neuromuscular disease caused by mutations in the dystrophin gene. The subcellular mechanisms of DMD remain poorly understood and there is currently no curative treatment available. Using a Caenorhabditis elegans model for DMD as a pharmacologic and genetic tool, we found that cyclosporine A (CsA) reduces muscle degeneration at low dose and acts, at least in part, through a mitochondrial cyclophilin D, CYN-1. We thus hypothesized that CsA acts on mitochondrial permeability modulation through cyclophilin D inhibition. Mitochondrial patterns and dynamics were analyzed, which revealed dramatic mitochondrial fragmentation not only in dystrophic nematodes, but also in a zebrafish model for DMD. This abnormal mitochondrial fragmentation occurs before any obvious sign of degeneration can be detected. Moreover, we demonstrate that blocking/delaying mitochondrial fragmentation by knocking down the fission-promoting gene drp-1 reduces muscle degeneration and improves locomotion abilities of dystrophic nematodes. Further experiments revealed that cytochrome c is involved in muscle degeneration in C. elegans and seems to act, at least in part, through an interaction with the inositol trisphosphate receptor calcium channel, ITR-1. Altogether, our findings reveal that mitochondria play a key role in the early process of muscle degeneration and may be a target of choice for the design of novel therapeutics for DMD. In addition, our results provide the first indication in the nematode that (i) mitochondrial permeability transition can occur and (ii) cytochrome c can act in cell death. },
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2012

Jean Giacomotto, Laurent Ségalat, Maïté Carre-Pierrat, Kathrin Gieseler
Caenorhabditis elegans as a chemical screening tool for the study o f neuromuscular disorders. Manual and semi-automated methods Journal Article
In: Methods, vol. 56, no. 1, pp. 103–113, 2012, ISSN: 1095-9130.
@article{pmid22041718,
title = {Caenorhabditis elegans as a chemical screening tool for the study o f neuromuscular disorders. Manual and semi-automated methods},
author = {Jean Giacomotto and Laurent Ségalat and Maïté Carre-Pierrat and Kathrin Gieseler},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2012-Methods-Elegans-Giacomotto.pdf},
doi = {10.1016/j.ymeth.2011.10.010},
issn = {1095-9130},
year = {2012},
date = {2012-01-01},
urldate = {2012-01-01},
journal = {Methods},
volume = {56},
number = {1},
pages = {103--113},
abstract = {We previously reported the use of the cheap and fast-growing nematode Caenorhabditis elegans to search for molecules, which reduce muscle degeneration in a model for Duchenne Muscular Dystrophy (DMD). We showed that Prednisone, a steroid that is generally prescribed as a palliative treatment to DMD patients, also reduced muscle degeneration in the C. elegans DMD model. We further showed that this strategy could lead to the discovery of new and unsuspected small molecules, which have been further validated in a mammalian model of DMD, i.e. the mdx mouse model. These proof-of-principles demonstrate that C. elegans can serve as a screening tool to search for drugs against neuromuscular disorders. Here, we report and discuss two methodologies used to screen chemical libraries for drugs against muscle disorders in C. elegans. We first describe a manual method used to find drugs against DMD. We further present a semi-automated method, which is currently in use for the search of drugs against the Schwartz-Jampel Syndrome (SJS). Both assays are simple to implement and can be readily transposed and/or adapted to screens against other muscle/neuromuscular diseases, which can be modeled in the worm. Finally we discuss, with respect to our experience and knowledge, the different parameters that have to be taken into account before choosing one or the other method.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Joachim Djiotsa, Vincianne Verbruggen, Jean Giacomotto, Minaka Ishibashi, Elisabeth Manning, Silke Rinkwitz, Isabelle Manfroid, Marianne L Voz, Bernard Peers
Pax4 is not essential for beta-cell differentiation in zebrafish embryos but modulates alpha-cell generation by repressing arx gene expression Journal Article
In: BMC Dev Biol, vol. 12, pp. 37, 2012, ISSN: 1471-213X.
@article{pmid23244389,
title = {Pax4 is not essential for beta-cell differentiation in zebrafish embryos but modulates alpha-cell generation by repressing arx gene expression},
author = {Joachim Djiotsa and Vincianne Verbruggen and Jean Giacomotto and Minaka Ishibashi and Elisabeth Manning and Silke Rinkwitz and Isabelle Manfroid and Marianne L Voz and Bernard Peers},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2012-BMC-Pax-Giacomotto.pdf},
doi = {10.1186/1471-213X-12-37},
issn = {1471-213X},
year = {2012},
date = {2012-12-01},
urldate = {2012-12-01},
journal = {BMC Dev Biol},
volume = {12},
pages = {37},
abstract = {BACKGROUND: Genetic studies in mouse have demonstrated the crucial function of PAX4 in pancreatic cell differentiation. This transcription factor specifies β- and δ-cell fate at the expense of α-cell identity by repressing Arx gene expression and ectopic expression of PAX4 in α-cells is sufficient to convert them into β-cells. Surprisingly, no Pax4 orthologous gene can be found in chicken and Xenopus tropicalis raising the question of the function of pax4 gene in lower vertebrates such as in fish. In the present study, we have analyzed the expression and the function of the orthologous pax4 gene in zebrafish.
RESULTS: pax4 gene is transiently expressed in the pancreas of zebrafish embryos and is mostly restricted to endocrine precursors as well as to some differentiating δ- and ε-cells but was not detected in differentiating β-cells. pax4 knock-down in zebrafish embryos caused a significant increase in α-cells number while having no apparent effect on β- and δ-cell differentiation. This rise of α-cells is due to an up-regulation of the Arx transcription factor. Conversely, knock-down of arx caused to a complete loss of α-cells and a concomitant increase of pax4 expression but had no effect on the number of β- and δ-cells. In addition to the mutual repression between Arx and Pax4, these two transcription factors negatively regulate the transcription of their own gene. Interestingly, disruption of pax4 RNA splicing or of arx RNA splicing by morpholinos targeting exon-intron junction sites caused a blockage of the altered transcripts in cell nuclei allowing an easy characterization of the arx- and pax4-deficient cells. Such analyses demonstrated that arx knock-down in zebrafish does not lead to a switch of cell fate, as reported in mouse, but rather blocks the cells in their differentiation process towards α-cells.
CONCLUSIONS: In zebrafish, pax4 is not required for the generation of the first β- and δ-cells deriving from the dorsal pancreatic bud, unlike its crucial role in the differentiation of these cell types in mouse. On the other hand, the mutual repression between Arx and Pax4 is observed in both mouse and zebrafish. These data suggests that the main original function of Pax4 during vertebrate evolution was to modulate the number of pancreatic α-cells and its role in β-cells differentiation appeared later in vertebrate evolution.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
RESULTS: pax4 gene is transiently expressed in the pancreas of zebrafish embryos and is mostly restricted to endocrine precursors as well as to some differentiating δ- and ε-cells but was not detected in differentiating β-cells. pax4 knock-down in zebrafish embryos caused a significant increase in α-cells number while having no apparent effect on β- and δ-cell differentiation. This rise of α-cells is due to an up-regulation of the Arx transcription factor. Conversely, knock-down of arx caused to a complete loss of α-cells and a concomitant increase of pax4 expression but had no effect on the number of β- and δ-cells. In addition to the mutual repression between Arx and Pax4, these two transcription factors negatively regulate the transcription of their own gene. Interestingly, disruption of pax4 RNA splicing or of arx RNA splicing by morpholinos targeting exon-intron junction sites caused a blockage of the altered transcripts in cell nuclei allowing an easy characterization of the arx- and pax4-deficient cells. Such analyses demonstrated that arx knock-down in zebrafish does not lead to a switch of cell fate, as reported in mouse, but rather blocks the cells in their differentiation process towards α-cells.
CONCLUSIONS: In zebrafish, pax4 is not required for the generation of the first β- and δ-cells deriving from the dorsal pancreatic bud, unlike its crucial role in the differentiation of these cell types in mouse. On the other hand, the mutual repression between Arx and Pax4 is observed in both mouse and zebrafish. These data suggests that the main original function of Pax4 during vertebrate evolution was to modulate the number of pancreatic α-cells and its role in β-cells differentiation appeared later in vertebrate evolution.
2010

Jean Giacomotto, Laurent Ségalat
High-throughput screening and small animal models, where are we? Journal Article
In: Br J Pharmacol, vol. 160, no. 2, pp. 204–216, 2010, ISSN: 1476-5381.
@article{pmid20423335,
title = {High-throughput screening and small animal models, where are we?},
author = {Jean Giacomotto and Laurent Ségalat},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2010-BJPharmacol-HTS-AnimalModels.pdf},
doi = {10.1111/j.1476-5381.2010.00725.x},
issn = {1476-5381},
year = {2010},
date = {2010-05-01},
urldate = {2010-05-01},
journal = {Br J Pharmacol},
volume = {160},
number = {2},
pages = {204--216},
abstract = {Current high-throughput screening methods for drug discovery rely on the existence of targets. Moreover, most of the hits generated during screenings turn out to be invalid after further testing in animal models. To by-pass these limitations, efforts are now being made to screen chemical libraries on whole animals. One of the most commonly used animal model in biology is the murine model Mus musculus. However, its cost limit its use in large-scale therapeutic screening. In contrast, the nematode Caenorhabditis elegans, the fruit fly Drosophila melanogaster, and the fish Danio rerio are gaining momentum as screening tools. These organisms combine genetic amenability, low cost and culture conditions that are compatible with large-scale screens. Their main advantage is to allow high-throughput screening in a whole-animal context. Moreover, their use is not dependent on the prior identification of a target and permits the selection of compounds with an improved safety profile. This review surveys the versatility of these animal models for drug discovery and discuss the options available at this day.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2009
Benjamin J Blaise, Jean Giacomotto, Mohamed N Triba, Pierre Toulhoat, Martial Piotto, Lyndon Emsley, Laurent Ségalat, Marc-Emmanuel Dumas, Bénédicte Elena
Metabolic profiling strategy of Caenorhabditis elegans by whole-organism nuclear magnetic resonance Journal Article
In: J Proteome Res, vol. 8, no. 5, pp. 2542–2550, 2009, ISSN: 1535-3893.
@article{pmid19267476,
title = {Metabolic profiling strategy of Caenorhabditis elegans by whole-organism nuclear magnetic resonance},
author = {Benjamin J Blaise and Jean Giacomotto and Mohamed N Triba and Pierre Toulhoat and Martial Piotto and Lyndon Emsley and Laurent Ségalat and Marc-Emmanuel Dumas and Bénédicte Elena},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/RMN-Proteome-Hi-Res.pdf},
doi = {10.1021/pr900012d},
issn = {1535-3893},
year = {2009},
date = {2009-05-01},
urldate = {2009-05-01},
journal = {J Proteome Res},
volume = {8},
number = {5},
pages = {2542--2550},
abstract = {In this study, we present a methodology for metabotyping of C. elegans using 1H high resolution magic angle spinning (HRMAS) whole-organism nuclear magnetic resonance (NMR). We demonstrate and characterize the robustness of our metabolic phenotyping method, discriminating wild-type N2 from mutant sod-1(tm776) animals, with the latter being an otherwise silent mutation, and we identify and quantify several confounding effects to establish guidelines to ensure optimal quality of the raw data across time and space. We monitor the sample stability under experimental conditions and examine variations arising from effects that can potentially confuse the biological interpretation or prevent the automation of the protocol, including sample culture (breeding of the worms by two biologists), sample preparation (freezing), NMR acquisition (acquisition by different spectroscopists, acquisition in different facilities), and the effect of the age of the animals. When working with intact model organisms, some of these exogenous effects are shown to be significant and therefore require control through experimental design and sample randomization.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Jean Giacomotto, Cordula Pertl, Caroline Borrel, Maggie C Walter, Stefanie Bulst, Bob Johnsen, David L Baillie, Hanns Lochmüller, Christian Thirion, Laurent Ségalat
Evaluation of the therapeutic potential of carbonic anhydrase inhibitors in two animal models of dystrophin deficient muscular dystrophy Journal Article
In: Hum Mol Genet, vol. 18, no. 21, pp. 4089–4101, 2009, ISSN: 1460-2083.
@article{pmid19648295,
title = {Evaluation of the therapeutic potential of carbonic anhydrase inhibitors in two animal models of dystrophin deficient muscular dystrophy},
author = {Jean Giacomotto and Cordula Pertl and Caroline Borrel and Maggie C Walter and Stefanie Bulst and Bob Johnsen and David L Baillie and Hanns Lochmüller and Christian Thirion and Laurent Ségalat},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2009-HMG-Sulfonamide.pdf},
doi = {10.1093/hmg/ddp358},
issn = {1460-2083},
year = {2009},
date = {2009-11-01},
urldate = {2009-11-01},
journal = {Hum Mol Genet},
volume = {18},
number = {21},
pages = {4089--4101},
abstract = {Duchenne Muscular Dystrophy is an inherited muscle degeneration disease for which there is still no efficient treatment. However, compounds active on the disease may already exist among approved drugs but are difficult to identify in the absence of cellular models. We used the Caenorhabditis elegans animal model to screen a collection of 1000 already approved compounds. Two of the most active hits obtained were methazolamide and dichlorphenamide, carbonic anhydrase inhibitors widely used in human therapy. In C. elegans, these drugs were shown to interact with CAH-4, a putative carbonic anhydrase. The therapeutic efficacy of these compounds was further validated in long-term experiments on mdx mice, the mouse model of Duchenne Muscular Dystrophy. Mice were treated for 120 days with food containing methazolamide or dichlorphenamide at two doses each. Musculus tibialis anterior and diaphragm muscles were histologically analyzed and isometric muscle force was measured in M. extensor digitorum longus. Both substances increased the tetanic muscle force in the treated M. extensor digitorum longus muscle group, dichlorphenamide increased the force significantly by 30%, but both drugs failed to increase resistance of muscle fibres to eccentric contractions. Histological analysis revealed a reduction of centrally nucleated fibers in M. tibialis anterior and diaphragm in the treated groups. These studies further demonstrated that a C. elegans-based screen coupled with a mouse model validation strategy can lead to the identification of potential pharmacological agents for rare diseases.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2007

Benjamin J Blaise, Jean Giacomotto, Bénédicte Elena, Marc-Emmanuel Dumas, Pierre Toulhoat, Laurent Ségalat, Lyndon Emsley
Metabotyping of Caenorhabditis elegans reveals latent phenotypes Journal Article
In: Proc Natl Acad Sci U S A, vol. 104, no. 50, pp. 19808–19812, 2007, ISSN: 1091-6490.
@article{pmid18077412,
title = {Metabotyping of Caenorhabditis elegans reveals latent phenotypes},
author = {Benjamin J Blaise and Jean Giacomotto and Bénédicte Elena and Marc-Emmanuel Dumas and Pierre Toulhoat and Laurent Ségalat and Lyndon Emsley},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/2007-RMN-PNAS.pdf},
doi = {10.1073/pnas.0707393104},
issn = {1091-6490},
year = {2007},
date = {2007-12-01},
urldate = {2007-12-01},
journal = {Proc Natl Acad Sci U S A},
volume = {104},
number = {50},
pages = {19808--19812},
abstract = {Assigning functions to every gene in a living organism is the next challenge for functional genomics. In fact, 85-90% of the 19,000 genes of the nematode Caenorhabditis elegans genome do not produce any visible phenotype when inactivated, which hampers determining their function, especially when they do not belong to previously characterized gene families. We used (1)H high-resolution magic angle spinning NMR spectroscopy ((1)H HRMAS-NMR) to reveal the latent phenotype associated to superoxide dismutase (sod-1) and catalase (ctl-1) C. elegans mutations, both involved in the elimination of radical oxidative species. These two silent mutations are significantly discriminated from the wild-type strain and from each other. We identify a metabotype significantly associated with these mutations involving a general reduction of fatty acyl resonances from triglycerides, unsaturated lipids being known targets of free radicals. This work opens up perspectives for the use of (1)H HRMAS-NMR as a molecular phenotyping device for model organisms. Because it is amenable to high throughput and is shown to be highly informative, this approach may rapidly lead to a functional and integrated metabonomic mapping of the C. elegans genome at the systems biology level.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2006

Maité Carre-Pierrat, Marie-Christine Mariol, Lucie Chambonnier, Aurélie Laugraud, Fabienne Heskia, Jean Giacomotto, Laurent Ségalat
Blocking of striated muscle degeneration by serotonin in C. elegans Journal Article
In: J Muscle Res Cell Motil, vol. 27, no. 3-4, pp. 253–258, 2006, ISSN: 0142-4319.
@article{pmid16791712,
title = {Blocking of striated muscle degeneration by serotonin in C. elegans},
author = {Maité Carre-Pierrat and Marie-Christine Mariol and Lucie Chambonnier and Aurélie Laugraud and Fabienne Heskia and Jean Giacomotto and Laurent Ségalat},
url = {http://giacomottolab.com/wp-content/uploads/2022/04/Sero1.pdf},
doi = {10.1007/s10974-006-9070-9},
issn = {0142-4319},
year = {2006},
date = {2006-01-01},
urldate = {2006-01-01},
journal = {J Muscle Res Cell Motil},
volume = {27},
number = {3-4},
pages = {253--258},
abstract = {Prevention of muscle fiber degeneration is a key issue in the treatment of muscular dystrophies such as Duchenne Muscular Dystrophy (DMD). It is widely postulated that existing pharmaceutical compounds might potentially be beneficial to DMD patients, but tools to identify them are lacking. Here, by using a Caenorhabditis elegans model of dystrophin-dependent muscular dystrophy, we show that the neurohormone serotonin and some of its agonists are potent suppressors of muscle degeneration. Inhibitors of serotonin reuptake transporters, which prolong the action of endogenous serotonin, have a similar effect. Moreover, reduction of serotonin levels leads to degeneration of non-dystrophic muscles. Our results demonstrate that serotonin is critical to C. elegans striated muscles. These findings reveal a new function of serotonin in striated muscles.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}



